1. Energy : Energy is the ability to do work. It can be viewed as an ability to cause changes. Energy can be found in many things and takes many forms.
1.1 Various forms of energy :
a) Potential Energy : The energy possessed by a body by virtue of its configuration or position is called as potential energy. It is a kind of stored energy.
b) Kinetic Energy : The energy possessed by a body by virtue of its state of motion is called as potential energy. The energy possessed by a body moving in a straight line is called translational kinetic energy. The energy possessed by a rotating body is called rotational kinetic energy. The energy possessed by a vibrating body is called vibrational kinetic energy.
c) Mechanical Energy : When potential and kinetic energy take place together, one after another then it is called as mechanical energy. It is also the sum of potential and kinetic energy.
d) Heat Energy : The energy released from burning action is called as heat energy.
e) Light Energy : The radiation produced by light in the visible spectrum is called as light energy.
g) Sound Energy : The energy possessed by a vibrating body is called as sound energy.
f) Electrical Energy : The energy possessed by an electron that conducts electric current while moving through an electric wire is called as electric energy.
1.2 Some other forms of energy that are used by mankind :-
a) Hydroelectricity : Energy generated from flowing water.
b) Magnetic Energy : The energy possessed by a magnet.
c) Wind Energy : The energy possessed by moving air.
d) Chemical Energy : The energy possessed by fuels and chemicals.
e) Thermal Energy : Energy stored in hot water.
f) Nuclear Energy : Energy stored in the nucleus of an atom.
2. Thermodynamics :
The name Thermodynamics comes from the Greek words Therme (Heat) and Dynamics (Force, Power). Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. It is science of energy and its interaction with matter.
2.1 Classical Thermodynamics ( Macroscopic view) : Classical thermodynamics does not require knowledge of the behaviour of individual molecules of the substance.
2.2 Statistical Thermodynamics ( Microscopic view ) : Statistical thermodynamics require knowledge of the behaviour of individual molecules of the substance.
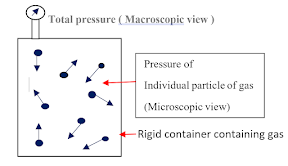 |
| Fig. 1.1 : Macroscopic and Microscopic View |
3. Thermodynamic System : A matter or a region in the space on which attention is given for study is called as system. The mass or a region outside the system is called as surrounding. The real or imaginary surface that separates the system from its surrounding is called as boundary. If the system and surrounding kept together then it called as universe.
 |
| Fig. 1.2 : Thermodynamic System |
 |
| Fig. 1.3 : Open System |
a) Open System : A system, in which both energy
and mass can cross the boundaries of the system
is called as an open system or control volume.
Boundaries of control volume is called as control surfaces.
Example: A turbine, A water heater, car radiator,...etc.
b) Closed System : A system, in which only energy can cross the boundaries of the system, but mass in the system remains constant, is called as closed system or control mass.
Example: Refrigerator, Thermometer, Electric bulb,...etc.
 |
| Fig. 1.4 : Closed System |
Example: Thermos flask, Universe,...etc.
 |
| Fig. 1.5 : Isolated System |
4. Properties of the System : The properties of system is a measurable characteristics which describe the system.
Example: Pressure, Temperature, Volume, Mass,...etc.
4.1 Types of Properties :
a) Intensive Property b) Extensive Property
A) Intensive Property : Intensive Properties are those that are independent of mass of the system.
Example: Temperature, Pressure and Density.
B) Extensive Property : Extensive Properties are those whose value depends on the mass or size of the system.
Example: Mass, Volume and Momentum.
** Some other properties **
1. Temperature : Temperature is the property of the system by virtue of which heat flows. It is the degree of hotness or coldness of a substance. It has unit degree centigrade (℃) or Kelvin (K).
2. Equilibrium : The word equilibrium means a balanced state.
3. Thermal Equilibrium : Two systems are in thermal equilibrium when their temperature is equal and no heat flows and no net flow of thermal energy between the system. Thermal equilibrium obeys the zeroth law of thermodynamics.
 |
| Fig. 1.6 : Thermal Equilibrium |
4. Mechanical Equilibrium : A system is in mechanical equilibrium with its surrounding, if there is no change in pressure at any point in the system and surrounding with time. i.e. pressure of system and surrounding is same.
 |
| Fig. 1.7 : Mechanical Equilibrium |
5. Chemical Equilibrium : A system is in chemical equilibrium with its surrounding, if the chemical composition of the system and surrounding does not change with time. ( i.e. no chemical reactions occur ).
6. Thermodynamic Equilibrium : If a system exists in thermal, mechanical and chemical equilibrium with its surrounding then the system is called as in thermodynamic equilibrium with its surrounding.
7. Quasi Equilibrium : Systems, in reality, does not undergo ideal thermodynamic equilibrium, but it goes through limiting equilibrium, which called as a quasi equilibrium.
8. Dead State : If the system is in thermodynamic equilibrium, it means the system is not interacting with its surrounding. Then the system is in dead state.
9. Internal Energy : Energy possessed by the system due to its molecular motion and configuration. Int views as the sum of kinetic and potential energies of the molecules.
 |
| Fig. 1.8 : Molecular Motion |
10. Process : Any changes that a system undergoes from one equilibrium state to another equilibrium state is called a process.
10.1 Quasi-Static or Quasi Equilibrium Process : In Latin quasi means ‘as if’. When a process proceeds in such a manner that, the system remains very very close to an equilibrium state at all the time, it is called as quasi-static or quasi equilibrium process.
10.2 Flow process : The processes generally occurring in the open system are flow processes in which mass entering and leaving through the boundary of an open system takes place.
Example: Process occurring in a steam turbine.
10.3 Non-flow process : The processes generally occurring in the closed system are non-flow processes in which constant mass within the definite boundary is undergoing a change of state.
Example: Process occurring in the refrigerator.
12. Cycle : A system said to have undergone a cycle if it returns to the initial state at the end of the process. A cyclic process carries the system through a cycle of stages, starting and being completed in some particular state.
To watch the video click here
.png)



.png)



No comments:
Post a Comment